BACKGROUND: High-risk(HR) cytogenetics are significantly enriched in MM with EMD , and studies consistently showed EMD and HR cytogenetics were associated with poor outcome. To date, there is no established approach for treatment of newly diagnosed multiple myeloma (NDMM) with EMD. Selinexor is a first-in-class Selective Inhibitor of Nuclear Export (SINE) compound that selectively binds and inactivates XPO1, which leads to accumulation of tumor suppressor proteins in the nucleus and re-activation of cell cycle regulators such as p53, FOXO, IkB. Meanwhile, by forcing the nuclear retention of the eIF4E protein, Selinexor reduces the cytoplasmic ribosomal translation of oncoproteins, including c-Myc, Bcl-2 and Bcl-6. In the STORM study(NCT02343042), 122 patients(pts) with penta-exposed, triple-class refractory multiple myeloma were enrolled to receive Selinexor combined with low-dose dexamethasone. 27 patients were diagnosed with EMD at baseline. The median age of patients was 64 years, the median prior therapies were 7, and 8 pts had high risk cytogenetics. Follow-up evaluation of plasma cell tumor was performed in 16 cases, and complete disappearance or shrinkage of extramedullary lesions was observed in 9 cases. It indicates that Selinexor has clinical activity in the treatment of plasma cell tumor or EMD. The treatment of MM with EMD or HR cytogenetics still faces great challenges, because there is no uniform guideline and consensus to recommend standard protocols, and prospective clinical studies are urgently needed to explore new treatment strategies.
METHODS: Eligible pts were aged≥18 years of NDMM with EMD or HR cytogenetics, and an Eastern Cooperative Oncology Group(ECOG) performance status of 0-2. For induction/consolidation(21-day cycles), pts received 8 cycles of SVRd (Selinexor 60 mg PO weekly, Bortezomib 1.3 mg/m2 SC days1, 4, 8, 11, Lenalidomide 25 mg PO days 1-14, and Dexamethasone 40 mg PO weekly. In maintenance (28-day cycles), pts received SR (Selinexor+ Lenalidomide) at least 2 years until disease progression, death or withdrawal. The primary endpoint was overall response rates (ORR) by end of induction. The secondary endpoints are complete response (CR), duration of response (DOR), progression free survival (PFS), overall survival (OS) and toxicity. This trial is registered with Chinese Clinical Trial Registry, number ChiCTR2200062860.
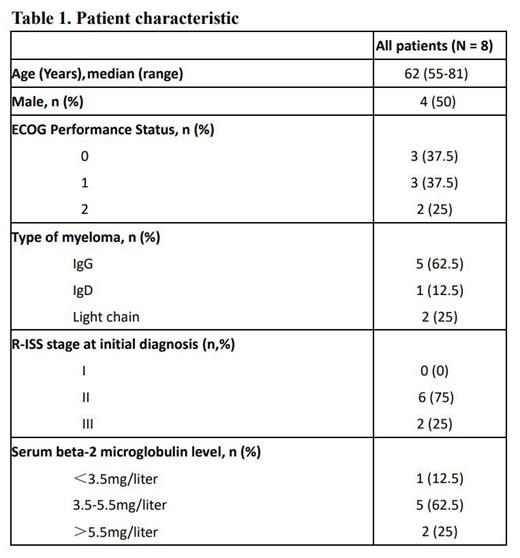
RESULTS: 8 pts with previously untreated NDMM were included from August 2022 to July 2023 in 3 hospitals. Median age was 62 (range 55-81). R-ISS stage 3 was present in 2 (25%) pts(Table 1). Based on inclusion criteria, 7 pts had EMD and 1 patient had HR cytogenetic, including 17p deletion (n=2, 25%), 1q21 gain(n=2, 25%). According to IMWG criteria, the ORR of 8 pts with NDMM was 100%, including 2 stringent complete response(sCR), 3 CR, 2 very good partial response (VGPR) and 1 partial response (PR). 1 patient evaluated with sCR at the end of the induction period was evaluated with MRD negativity after autologous stem cell transplantation (ASCT). Median time to first response in patients with a partial response or better was 1 months (1-2). This result established that SVRd regimen has a prospective response for MM with EMD or HR cytogenetic. The most common grade 3-4 treatment-emergent adverse events (occurring in ≥10% of pts) were thrombocytopenia. The most common treatment-related adverse events were grade 1 or 2, including nausea (25%), fatigue (25%), and anorexia (25%). Overall, the severe toxicities are manageable.
CONCLUSIONS: SVRd as induction prior ASCT is safe and allows deep responses in NDMM patients with EMD or HR cytogenetic profile. It is worthy of further study based on the preliminary efficacy results. Longer-term results still require more pts have been enrolled and further follow-up.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal